Last Updated on August 15, 2023 by Heather Hart, ACSM EP, CSCS
Everyone has their pet peeves in life. For some, it’s the toilet paper roll facing the wrong direction. For others, it’s the sound of a person chewing with their mouth open. For me, it’s the woeful misunderstanding of lactate that runs rampant in the fitness and running industry.
Which is extra unfortunate, because many of the misconceptions surrounding lactate were cleared up nearly forty years ago. But for whatever reason, these myths and misunderstandings have a strong hold on everyone from runners, to coaches, to personal trainers, and other experts in the industry.

Welcome to part two of my three part series discussing “everything runners need to know about lactate”. In this post, I’m covering 9 lactate misconceptions and lactic acid myths, that run (pun intended) rampant in the endurance community.
If you’re not entire sure what this “lactate” business is all about in the first place, I highly recommend checking out part one: The Runner’s Guide to Understanding Lactate before reading this post.
9 Common Lactate / Lactic Acid Myths Among Runners:
Forewarning: it’s kind of impossible to talk about biochemistry without getting quite a bit “sciencey”. And trust me, I get it, it can be overwhelming and confusing. So for those who want the highlights…I’ve literally highlighted them for you.
Those of you who want to dive a little deeper into the “why” behind these misconceptions …well I’ve covered that too.
Here we go:
1. Lactic Acid and Lactate are Not the Same Thing
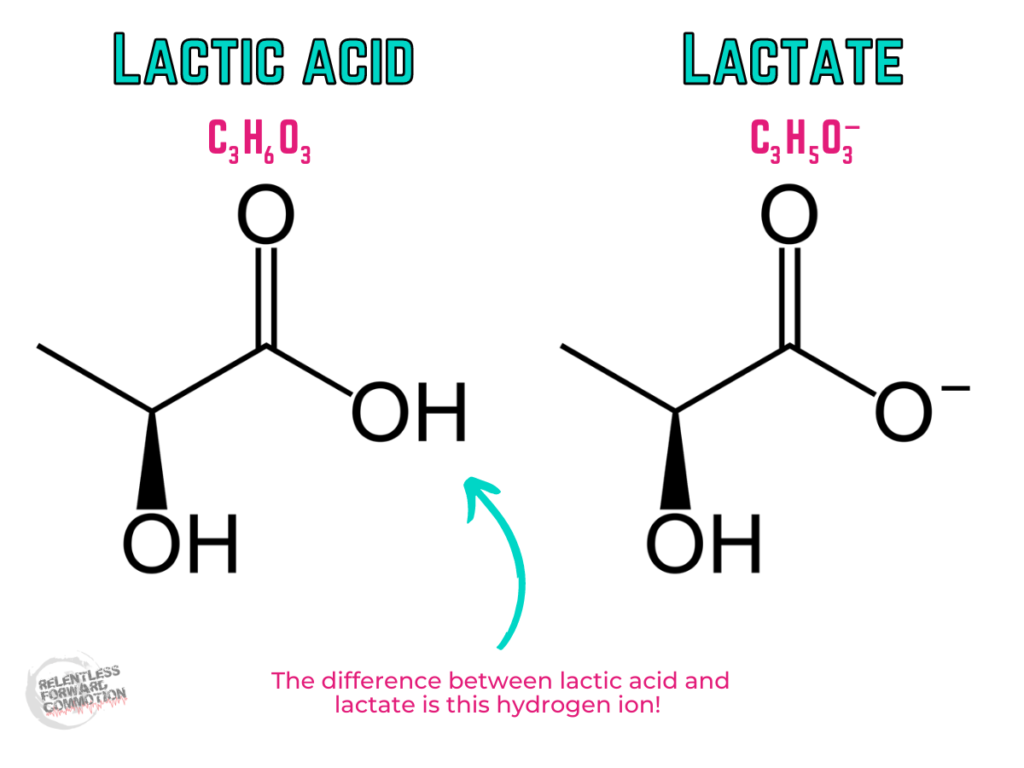
Lactate and lactic acid are simply not the same chemical compound, structurally, therefore, they are not the “same thing”.
To many this may sound like silly science semantics, like arguing Pepsi vs. Coke: they’re both colas. But I’d argue it’s more like cow’s milk vs. rice milk: not the same thing at all.
Lactic acid is an organic compound and a carboxylic acid. The chemical formula of lactic acid is C3H6O3
Lactate ion is the conjugated base of lactic acid. The chemical formula of lactate is C3H5O3–.
In order to be an acid, a molecule needs to have an extra hydrogen ion (proton) to donate.
2. Lactic Acid Doesn’t Exist in the Human Body
The human body operates in a very narrow pH range, that is not compatible for lactic acid to exist. So you simply do not have lactic acid floating around in your blood stream.
Now, for a long time it was thought that end product of anaerobic glycolysis is the conversion of pyruvic acid to lactic acid. Because lactic acid is a weak base that cannot exist in the body’s pH environment, the hydrogen ion is immediately dropped (disassociated), leaving a separate hydrogen ion and lactate molecule.
But newer research suggest that this doesn’t happen either, and that lactic acid never even forms to begin with.
Here’s what we now know:
- The phosphoglycerate kinase reaction (the process of breaking down glucose into pyruvate) involves the transfer of a phosphate leaving a carboxylate group, resulting in no proton (H+) existing to attach to (and then dissociate from) lactate.
- The lactate dehydrogenase reaction (the process of converting pyruvate into lactate) itself consumes protons. So the creation of lactate actually helps REMOVE hydrogen ions, rather than creates them.

While this is still a pretty highly debated topic in the world of exercise science, it appears more and more researchers are agreeing that lactic acid is not produced in muscle cells, and that it is not present in meaningful concentrations (9)
3. Lactate Does Not Cause Muscle Burning, Cramps, or Fatigue
Lactate is not directly responsible for that burning you feel in your legs at the end of a 5K, the utter fatigue felt at the end of a tempo run, or feeling like your legs will lock up in cramps at the end of a bunch of 100 meter repeats. It’s actually hydrogen ion’s fault.
Very early research (we’re talking in the 1920’s, initially performed on frog legs) showed that high intensity exercise (like heavy lifting or a hard run) consistently produced high levels of “lactic acid”.
At the same time, the high levels of “lactic acid” were accompanied by muscular fatigue, burning muscles, muscular failure, cramps, and general feelings of malaise.
Naturally, it was assumed the “lactic acid” was at fault.
It was believed that “lactic acid” was a waste product of cellular metabolism, and that lactate was responsible for changing the pH of the blood, and wrecking havoc within the body.
Many years later, even when the distinction between lactate and hydrogen ions was made, lactate was still cited as the cause for muscular fatigue and soreness.
We now know that the accumulation of hydrogen ions changes the pH of the muscle tissue cells and blood. And this drop in pH is akin to getting a shoelace caught in your bicycle gears: everything starts to slow down until suddenly you crash.
So Where Do the Hydrogen Ions Come From?
“But wait, Heather…didn’t you just say there isn’t a hydrogen ion to dissociate from lactate? But you’re telling me hydrogen ions are responsible for those hard effort sensations?”
I know, it’s a little confusing.
Hydrogen ions exists as a byproduct of cellular metabolism. How hydrogen ions are produced is super complex. Some of the ways we produce hydrogen ions include:
- General breakdown of ATP (ATP hydrolysis). The actual act of splitting ATP, which is what directly provides”energy” for cells, leaves behind the products of ADP, an inorganic phonate molecule, and a hydrogen ion.
- Strong Ion Difference: the body’s balance between positively charged ions (cations) and negatively charged ions (anions). Your body wants to maintain an equal balance (known as “electroneutrality”) between the two in order to maintain homeostasis.
But, this electroneutrality balance can be thrown off by intense exercise. First, cations like sodium and potassium are lost through sweat, and the accumulation of lactate (an anion) both lower the strong ion difference. As a result, your body will split water molecules, creating hydrogen ions that help regain the balance.
Point being? Hydrogen ion accumulation exists as a byproduct of cellular metabolism, NOT as a result of lactate production.
Lactic Acidosis vs. Metabolic Acidosis
Time for some more very important semantics! The drop in blood pH resulting in burning or fatigue is often referred to as “Lactic acidosis”.
But, as we’ve established already, it’s not the lactate causing a drop in pH (“acidosis”), it’s actually the hydrogen ions produced in various stages of metabolism, or “metabolic acidosis”.
“Lactic acidosis” is a misnomer.

The debate does exist as to whether or not lactate indirectly causes cellular acidosis exists, due to the change in strong ion difference with an accumulation of lactate, and the resulting increase in hydrogen ions. But, even in this example, it’s still the hydrogen ions causing the acidosis, not lactate directly.
4. Lactate is Not a Waste Product
Lactate is not a waste product of anaerobic glycolysis. Rather, it’s a purposefully created molecule that is incredibly useful as a source of energy, a buffer to help maintain electroneutrality, and a useful cell signaling molecule.
Once again this misconception stemmed from very early research, and hasn’t let go of it’s stronghold among the general population, despite research dating as far back as the 1970’s demonstrating that lactate was not a waste product.
Let’s look at all the awesome things lactate does:
Lactate as an Intracellular Energy Source
Lactate can be moved into the mitochondria of the muscle cell where it was created, or adjacent type I muscle fibers (where aerobic glycolysis occurs) and converted BACK into pyruvate, which then enters the Krebs cycle, and can be oxidized into ATP.
Lactate as an Extracellular Energy Source
Lactate can be shuttled to other cells besides skeletal muscle to be used as a source of energy. In fact, the brain, heart, liver, kidneys, and adipose tissue (fat) can all use lactate as an energy substrate, through a process called gluconeogenesis (forming glucose from non-carbohydrate sources).
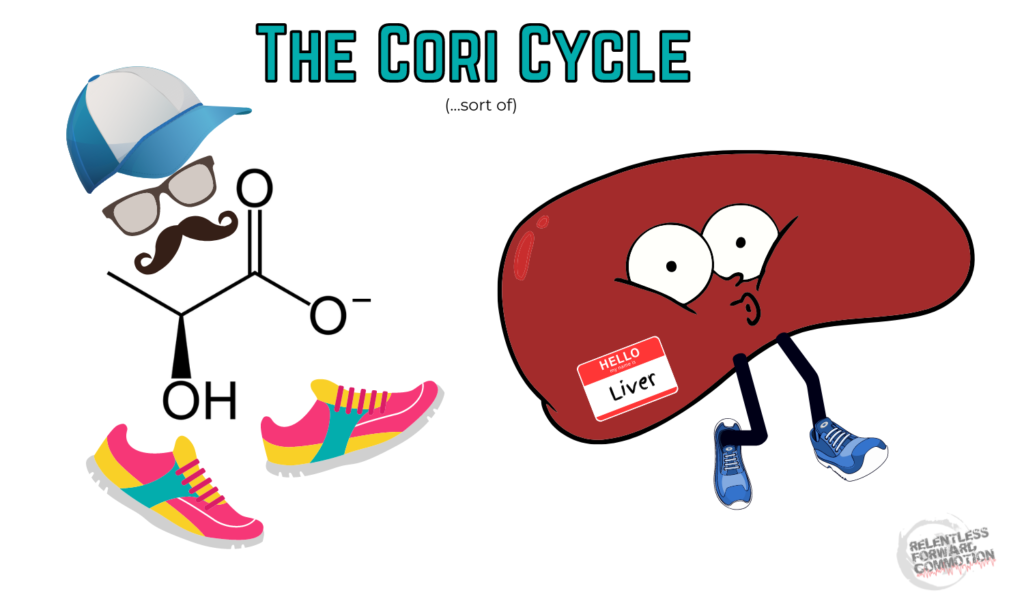
The Cori Cycle
Lactate can be transported from muscle cells, into the blood, and shuttled to the liver where it is converted to glucose. That glucose can either be immediately run back through glycolysis to produce energy, OR it can be converted to glycogen and stored in the liver, to be used later.
Lactate as a Buffer
Lactate acts as a buffer to help try and keep our body happily in homeostasis as the stress of exercise increases.
Lactate Buffers Pyruvate: When we exercise at a higher intensity, pyruvate production occurs at rates that exceeds the capacity of the mitochondria to take in that pyruvate, and shuttle it through the Krebs cycle & electron transport chain.
So in order to prevent a jam in that energy production cycle and, as much pyruvate as possible must be removed from the cytosol. The creation of lactate through the lactate dehydrogenase reaction
Lactate Buffers Hydroge Ions: As mentioned earlier in this post, the lactate dehydrogenase reaction (the process of converting pyruvate into lactate) itself consumes protons. So the creation of lactate actually helps buffer hydrogen ions.
Cell Signaling Molecule
Research shows that lactate acts as a cell signaling molecule, helping transmit messages and information between cells in your body, and may aid in:
- Helping the brain after trauma
- Improving cardiac function in damaged tissue
- Active participation in wound healing with increased development of new blood vessels
- Neuroprotection through anti-inflammatory activities (8)
5. Lactate Does Not Cause DOMS
Lactate is not responsible for the post workout or next day soreness many runners experience after a hard workout. That soreness is referred to as “Delayed Onset Muscle Soreness“, or “DOMS”, and is caused by micro trauma to muscle and connective tissues, as well as the subsequent inflammation associated with the healing of that tissue.
Basically, eccentric muscle contractions (like what’s going on in your quads while running steep downhills) causes the myosin heads of a muscle filament (the part responsible for the actual movement in a muscle contraction) to tear.

Sounds painful, right? Well if you’ve ever experienced DOMS, you can confirm…it (eventually) hurts. But the good news is, this is a totally natural response to training and a part of the whole muscle remodeling process. In short, it’s how you get stronger.
But once again, lactate is not to blame.
6. You Don’t Need to “Flush Out” Lactate After a Run
Despite what you may have seen on social media, lactate does not need to be stretched, foam rolled, or otherwise “flushed out” of your body after a workout. Your body will take care of it on its own.
Endless studies have shown that blood lactate concentrations typically return to pre exercise values within an hour after activity.
In fact, the fitter you are, the faster you’ll clear lactate from your body. Research shows that both endurance and anaerobically trained athletes have faster lactate clearance rates than untrained people. (6)
Now, that’s not to say that active recovery isn’t beneficial: it is. Light activity during the post exercise period has been shown to increase lactate clearance rates. And if it feels good as a part of your post-workout routine? Then absolutely go for it.
But, if you do nothing other than go sit on your couch and watch 4 hours of Netflix after a run (or simply don’t have time after training), your body is STILL going to eventually clear lactate on it’s own.

7. Easy, Aerobic Runs Help Build Lactate Clearance Capabilities
Pretty much every coach in the running industry touts tempo runs as the number one way to improve your lactate threshold (hell, I did too for a long time).
And there is some truth to this: studies suggest that training at intensities near or above the lactate threshold or onset of blood lactate accumulation will increase the intensity at which you can run before lactate accumulation begins to occur.
However, slower, easy, aerobic based runs are highly effective at improving your lactate clearance capabilities.
We now understand that much of lactate clearance occurs in the mitochondria of slow twitch (type I) muscle fibers. And lower intensity exercise (think: base building or easy long runs) helps increase the mitochondria density in type I fibers. This means you’ll not only be able to create more ATP aerobically with more mitochondria, but also clear lactate faster.
Further, lower intensity exercise can help improve the amount of MCT-1 (monocarboxylate) transporters, which help shuttle lactate to other parts of the body, and mLDH (lactate dehydrogenase) enzymes, which help catalyze lactate back to pyruvate again.
8. Lactate Threshold is a Better Indicator of Fitness than VO2Max
Endurance athlete’s tend to focus on VO2max as an indicator of fitness. However, VO2 max is greatly dependent upon genetics, and for many, minimally trainable, making it a better indicator of maximal potential.
Lactate threshold is better indicator of current fitness, and can be greatly influenced by training.

Unfortunately, both are kind of difficult to truly test, as both need to be done in an exercise science or performance lab setting.
9. Low Oxygen Availability Isn’t The Only Time We Create Lactate
If you think during intense exercise, like hard hill repeats, or 1RM deadlifts, is the only time we create lactate: think again.
Our bodies are constantly creating – and clearing – lactate. Even while we sleep!
The normal amount of lactate present in the blood during rest is around 0.5 to 2.2 mmol/L (“millimoles per liter”), meaning, there’s always some lactate coming and going.
Lactate production is influenced by muscle fiber type being used, rate of glycolysis, ATP hydrolysis, and rate of hydrogen ion increases, not simply the availability of oxygen in muscle tissue.
Lactate Myths – Takeaway Points:
I hope if you’ve taken away anything from this post, it’s that:
- Lactic acid doesn’t exist in the human body in any sort of notable concentration
- Lactate isn’t responsible for any of those “I hate running 400 m repeats” feelings of exhaustion or soreness, and
- Lactate is NOT a waste product!
Stay tuned for part 3 of this series, where we’ll talk about exactly what sort of workouts you can and should do to help improve lactate clearance.
Resources:
- Baker, J., & Grant, M.C., & Robergs, R. (2010). Interaction among Skeletal Muscle Metabolic Energy Systems during Intense Exercise. Journal of nutrition and metabolism. 2010. 905612. 10.1155/2010/905612.
- Brooks G. A. (2018). The Science and Translation of Lactate Shuttle Theory. Cell metabolism, 27(4), 757–785. https://doi.org/10.1016/j.cmet.2018.03.008
- Donovan, C. M., & Brooks, G. A. (1983). Endurance training affects lactate clearance, not lactate production. The American journal of physiology, 244(1), E83–E92. https://doi.org/10.1152/ajpendo.1983.244.1.E83
- Ferguson, B. S., Rogatzki, M. J., Goodwin, M. L., Kane, D. A., Rightmire, Z., & Gladden, L. B. (2018). Lactate metabolism: historical context, prior misinterpretations, and current understanding. European journal of applied physiology, 118(4), 691–728. https://doi.org/10.1007/s00421-017-3795-6
- Gollnick, P. D., Bayly, W. M., & Hodgson, D. R. (1986). Exercise intensity, training, diet, and lactate concentration in muscle and blood. Medicine and science in sports and exercise, 18(3), 334–340. https://doi.org/10.1249/00005768-198606000-00015
- Haff, G.G., & Triplett, T.N (2016). Essentials of Strength and Conditioning (4th ed.). Human Kinetics.
- Hall, M. M., Rajasekaran, S., Thomsen, T. W., & Peterson, A. R. (2016). Lactate: Friend or Foe. PM & R : the journal of injury, function, and rehabilitation, 8(3 Suppl), S8–S15. https://doi.org/10.1016/j.pmrj.2015.10.018
- Lee T. Y. (2021). Lactate: a multifunctional signaling molecule. Yeungnam University journal of medicine, 38(3), 183–193. https://doi.org/10.12701/yujm.2020.00892
- Lindinger, M. I., Kowalchuk, J. M., & Heigenhauser, G. J. (2005). Applying physicochemical principles to skeletal muscle acid-base status. American journal of physiology. Regulatory, integrative and comparative physiology, 289(3), R891–R910. https://doi.org/10.1152/ajpregu.00225.2005
- Merrells, R. J., Madon, S. B., Chivers, P. T., & Fournier, P. A. (2021). Nausea after Repeated Sprints: Is Lactic Acidosis Really the Culprit?. Medicine and science in sports and exercise, 53(9), 1865–1872. https://doi.org/10.1249/MSS.0000000000002667
- Rabinowitz, J.D., Enerbäck, S. Lactate: the ugly duckling of energy metabolism. Nat Metab 2, 566–571 (2020). https://doi.org/10.1038/s42255-020-0243-4
- Robergs, R. A., Ghiasvand, F., & Parker, D. (2004). Biochemistry of exercise-induced metabolic acidosis. American journal of physiology. Regulatory, integrative and comparative physiology, 287(3), R502–R516. https://doi.org/10.1152/ajpregu.00114.2004
- Stallknecht, B., Vissing, J., & Galbo, H. (1998). Lactate production and clearance in exercise. Effects of training. A mini-review. Scandinavian journal of medicine & science in sports, 8(3), 127–131. https://doi.org/10.1111/j.1600-0838.1998.tb00181.x
- Vucetic, V., Mozek, M., & Rakovac, M. (2015). Peak blood lactate parameters in athletes of different running events during low-intensity recovery after ramp-type protocol. Journal of strength and conditioning research, 29(4), 1057–1063. https://doi.org/10.1519/JSC.0000000000000725
Heather Hart is an ACSM certified Exercise Physiologist, NSCA Certified Strength and Conditioning Specialist (CSCS), UESCA certified Ultrarunning Coach, RRCA certified Running Coach, co-founder of Hart Strength and Endurance Coaching, and creator of this site, Relentless Forward Commotion. She is a mom of two teen boys, and has been running and racing distances of 5K to 100+ miles for over a decade. Heather has been writing and encouraging others to find a love for fitness and movement since 2009.

Leave a Reply